Author Affiliations
Comprehensive Cell Solutions, New York Blood Center Enterprises, 310 E 67th Street, New York, New York 10065
Introduction
Precision Cell Sourcing Across the Continuum of Needs
There is increasing interest in exploring cell-based therapies for the treatment of numerous conditions, such as hematologic malignancies and non-malignancies, immune disorders, neurological conditions, and more.1 Cellular therapies include chimeric antigen receptor (CAR) T cells or NK cells, gene-modified hematopoietic stem cells, mesenchymal stem cells, retinal pigment epithelial cells, B cells, and dendritic cells.2 Cell sourcing is the pivotal first step for the manufacture of advanced cellular therapies that harness the potential of cells to be leveraged as medicines. The careful identification, isolation, and collection of the target cell type(s) from a donor or a patient in an allogeneic therapy is critical, as it directly impacts the success and efficacy of downstream therapeutic engineering endeavors.
Apheresis
Apheresis is a medical procedure that uses a device to draw in whole blood and separate the blood components by density and/or size in order to collect a target element. The remaining blood components are then returned to the donor (Figure 1). To prevent whole blood from clotting while circulating in the apheresis device, an anticoagulant solution of acid citrate dextrose solution A (ACD-A) is added to the system at a rate determined by the patient’s total blood volume in order to optimize blood flow and reduce the risk of citrate-related hypocalcemia. Apheresis procedures encompass both therapeutic procedures to remove a diseased blood component in a patient (e.g., plasma exchange, red cell exchange, or cell depletion procedures) and cellular therapy collections in either a donor (allogeneic) or a patient (autologous) for bone marrow transplant or other cell- and gene-based therapies. For certain progenitor cells, a mobilization agent such as granulocyte colony-stimulating factor (G-CSF) or plerixafor is administered prior to the apheresis procedure to stimulate movement of the desired cells from the bone marrow compartment where they reside into the peripheral circulation.1
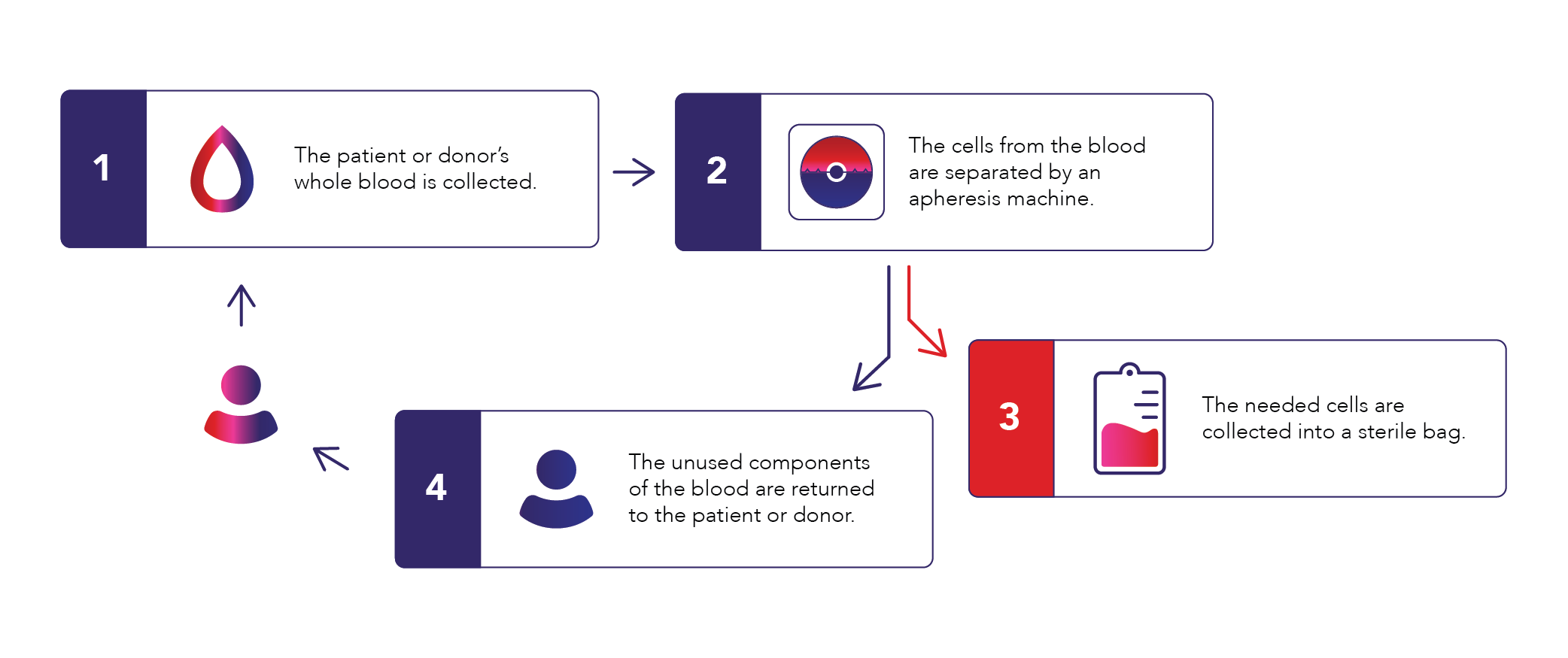
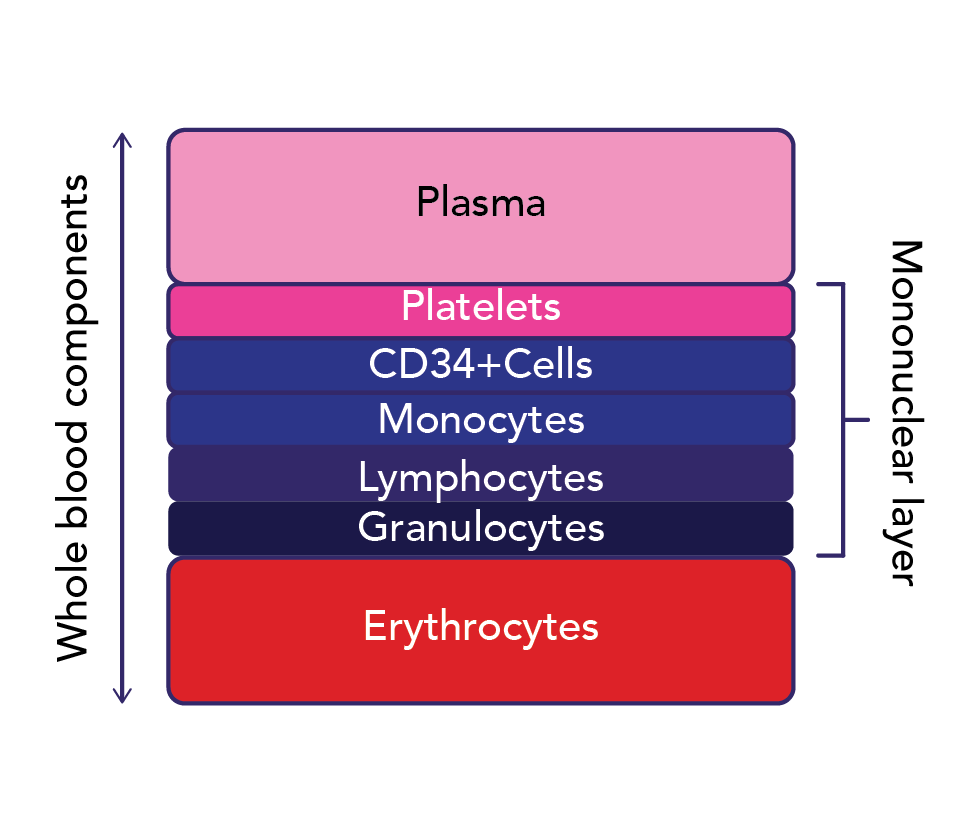
Cellular therapy collections can be targeted towards various cell types, but in the case of most cell- and gene-based therapies, the cells of interest are found in the mononuclear cell (MNC) layer (Figure 2).3 Such target cells include CD34+ hematopoietic progenitor cells (HPCs), lymphocytes, NK cells, and monocytes. CD34 is a marker of HPCs, which are progenitor cells that give rise to all blood components through differentiation. Reliable and precise collection of the target cell type by apheresis is key to both optimal donor safety and patient outcomes with cell- and gene-based therapies. Reliability ensures the target cell number is collected consistently; precision ensures that the target cells are collected with optimal purity to minimize the presence of undesired cells. Also critical is that validated and reliable processes are in place to send the collected product to the downstream manufacturing partner in a timely manner to ensure viability and optimal fitness for processing. This includes maintaining the collected product’s chain of identity and chain of custody, from the collection and processing facilities to the manufacturer to its final disposition into the patient.
Achieving Reliable and Precise Cell Therapy Collections
Background
Comprehensive Cell Solutions (CCS) is a division of New York Blood Center Enterprises (NYBCe) dedicated to cell and gene therapy-focused development and manufacturing. As discussed above, achieving the target cell dose via apheresis is critical to ensure the adequate number of cells are available for downstream processing and therapeutic manufacturing and, ultimately, treatment of the patient in need. Historically, collection centers have used various fixed parameters when collecting target cells, such as a preset number of liters of blood, a fixed number of total blood volumes, or predetermined duration of time. Several algorithms have been offered to predict the volume of blood to process on a per- patient basis to achieve a given target cell dose, but none have been adopted broadly.4 Barriers to adoption include reliance on linear regression for calculations, which may prove challenging to some collection centers. Furthermore, linear regression algorithms are prone to underestimate the volume needed to reach the target cell dose.4
Hematopoietic Progenitor Cell (CD34+) Collection
We have validated a simple prediction algorithm for CD34+ HPC collections, enabling the direct calculation of the minimum blood volume required to achieve the desired target dose and eliminating the need for linear regression (Figure 3).4 A calculator developed as part of this research can be found in the appendix of the aforementioned reference. In 93% of collections, the target CD34+ cell dose was achieved. While the algorithm was validated using allogeneic CD34+ HPC collections, we have applied the algorithm to autologous HPC collections with similar success. A pivotal factor contributing to the high success rate of this algorithm is its utilization of a collection center-specific first-quartile CD34+ collection efficiency (CE), effectively minimizing the risk of under-collection. Another factor contributing to success involves leveraging the donor’s peripheral blood MNC count before starting apheresis to tailor the collect flow rate (CFR) during the procedure, resulting in higher consistency of CD34+ CEs with similar rates of platelet, red cell, or granulocyte contamination.4 We use analogous strategies to obtain the same success rate of 93% with HPC collections that are followed by an immunomagnetic selection procedure, such as positive CD34+ selection.5 These practices decrease collection time and minimize overcollection, thus improving donor safety and donor experience.

T-Lymphocyte (CD3+ Cell) Collection
Similar to CD34+ HPCs, other MNC types are in demand for engineering immune effector cellular therapies such as CAR-T cells, CAR-NK cells, and dendritic cells. Similar to HPC collection, the CCS team has validated a prediction algorithm for lymphocyte collection to calculate the liters of blood to process for a given donor, without use of linear regression, to ensure adequate target cell yields (Figure 4). This approach leverages the pre-collection peripheral blood absolute lymphocyte count (ALC) to estimate the CD3+ count, thereby increasing the algorithm’s usability and cost-saving benefits. Use of the algorithm resulted in 93% of lymphocyte collections achieving the final target CD3+ cell dose.5 A calculator developed as part of this research can be found in the appendix of the aforementioned reference.

Optimizing Additional Technical Aspects of Collection Procedures
In addition to using patient-specific parameters to determine the liters required to achieve the target cell dose, other technical parameters of the collection procedure can be adjusted to optimize cell yield or purity.6 Based on our findings indicating that target cell CE decreases as peripheral blood concentration increases, we are collaborating with Terumo BCT to explore modifications to our MNC-driven collect flow rate algorithm, aiming to further enhance CEs. Stemming from our findings on the potential layering of various target cell types within the MNC layer (Figure 2), we are collaborating with Terumo BCT to refine our collection preference within the MNC layer, aiming for more precise targeting of the cell type of interest. Regarding cell purity, we found that a decreased inlet flow rate (IFR) is predictive of decreased RBC contamination, while an increased anticoagulation rate or ratio is predictive of decreased granulocyte contamination.3 Such retrospective analyses are fruitful avenues for continual optimization of collection procedures and processes to best serve patients and donors.
Sharing Expertise and Best Practices with Others
The key to excellence as a cell sourcing and apheresis partner lies in a commitment to ongoing research to continue advancing cell therapy collection practices, annual and as needed review of procedures and protocols, and dissemination of peer-reviewed findings, both our own and those of others, to the scientific and medical community. CCS engages in research and review of existing information to advance the field of cell sourcing. In addition to literature review on the optimization of product cell yield and product purity,1 other examples of CCS’ participation in sharing experience and perspectives that advance the field of cell sourcing include:

CCS Approach in Action: Innovations in Pediatric CAR T-Cell Therapy
CD19-directed CAR T-cell therapy has shown encouraging rates of remission in pediatric patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).10 MNC collections in very young patients can be challenging because the low IFR required, due to their low total blood volume increases the risk of line clotting when using standard ACD-1 rates and ratios. One method to overcome this issue is to use higher ACD-A infusion rates than recommended; however, this approach risks citrate toxicity and requires intensive calcium replacement monitoring. To circumvent this challenge, the CCS team used a combination of heparin, a different type of anticoagulant, and ACD-A anticoagulation in a 20-month-old, 7.5 kg patient with relapsing high-risk B-cell precursor ALL. The combination of these two agents enabled a safe increase of the IFR while minimizing the risk of citrate toxicity, thereby reducing the frequency of calcium monitoring required.11 Importantly, the use of heparin with ACD-A did not affect collection efficiency or increase granulocyte, platelet, or red cell contamination.6,11 This anticoagulation combination can be leveraged for mobilized HPC collections as well as non-mobilized MNC collections for immune effector cell therapy.
CCS Approach in Action: Innovations in Sickle Cell Disease
Optimizing Mobilization to Enhance Collection Efficiencies
Sickle cell disease (SCD) is a congenital hemoglobinopathy caused by a single base pair mutation in the β-globin gene. In low oxygen conditions, hemoglobin assumes a misshapen sickle-like form, resulting in increased red cell rigidity, activation of other cell types, and blood vessel occlusion. Various gene therapy approaches for SCD have recently been approved or are in clinical trials. Gene therapy for SCD currently requires mobilization from the bone marrow and apheresis collection of sufficient and high-quality autologous HPCs for ex vivo gene modification.
CCS was the first to complete pre-clinical studies for a clinical trial studying plerixafor as an alternative mobilizing agent for gene therapy in SCD,12,13 where first-line G-CSF is contraindicated due to its potential to induce life-threatening vaso-occlusion. Based on evidence suggesting that plerixafor dose escalation may enhance target cell yields,14 CCS also examined the safety and efficacy of escalating plerixafor from the standard dose of 240 ug/kg to a dose of 320 ug/kg in two patients with SCD (NCT02193191).15 The data generated suggests that such dose escalation may be safe and effective.
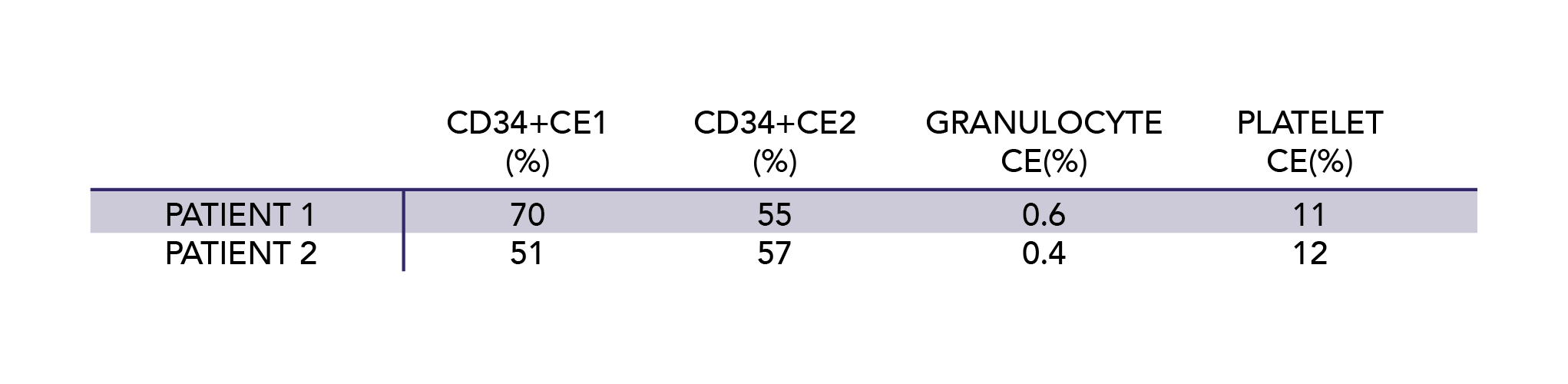
In order to mitigate MNC layer instability and low CD34+ CE reported in previous literature on HPC collection in SCD, we adjusted the collection process (timing the red cell exchange to the day prior to plerixafor mobilization) and the collection procedure (adding heparin anticoagulation and increasing the packing factor, a correlate of centrifugal speed) in the two clinical trial patients who underwent HPC collection (Table 1). We did not observe any issues with an unstable interface or low CD34+ CE in this study limited to two patients, and had notably low granulocyte and platelet contamination.16 Continued study of such adjustments in a larger cohort of patients may prove beneficial in the care of SCD patients.
Learning from Patient Outcomes
Currently FDA-approved gene therapies for SCD require or recommend at least one transfusion, preferably by red cell exchange, prior to HPC mobilization and collection to improve their safety and efficacy. CCS was the first to report in detail the risk of severe delayed hemolytic transfusion reactions (DHTR) with this red cell exchange requirement.17 Patients with SCD are at increased risk of red cell alloimmunization. One of the two patients who underwent HPC collection in the aforementioned clinical trial developed a severe DHTR complicated by a pulmonary embolism 5 days after uneventful red cell exchange, plerixafor mobilization, and HPC collection. This case illustrates the importance of raising awareness of disease-specific complications associated with apheresis.
As part of an ongoing mission to improve cell collection processes and ensure the safest outcomes for donors and patients, CCS is investigating alternatives to red cell transfusion as preparation prior to HPC mobilization and collection prior to gene therapy. Current efforts are centered on evaluating whether voxelotor, an FDA-approved drug for SCD that increases the affinity of Hb for oxygen and raises Hb, could act as a safe and efficacious alternative to transfusion.18 In a preliminary study, voxelotor increased the percentage of bone marrow hematopoietic stem cells (HSCs) comparably to transfusion, and upon subsequent plerixafor mobilization, the percentage of peripheral blood HSCs increased. Inflammatory biomarkers were not significantly increased post-plerixafor, suggesting the potential safety of this approach.18
CCS Approach in Action: Innovations in Difficult-to-Treat Hematologic Malignancies
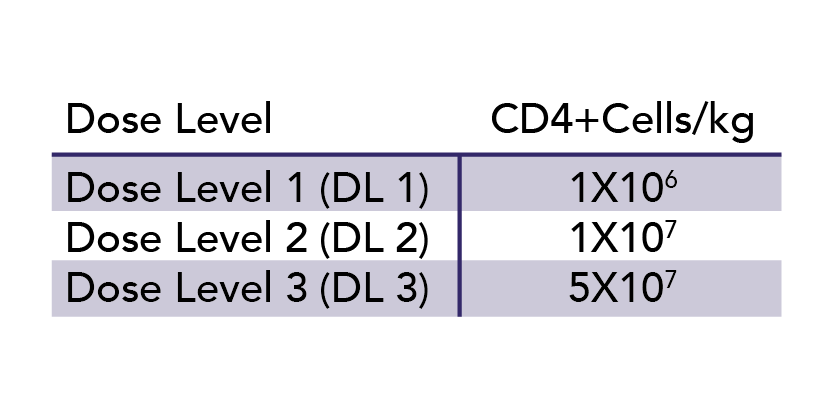
Despite the advancement of gene-modified immune effector cell therapy, both standard and modified allogeneic donor lymphocyte infusions (DLI) continue to be significant areas of investigation due to their relative resistance against immune exhaustion, in contrast to autologous lymphocytes. CCS’s recruitment of blood donors for MNC or HPC collection has contributed to partnered research in this area. In one recent study, CCS was able to recruit Class II HLA-mismatched but ABO- and CMV-compatible, donors for T-lymphocyte collection for all 9 patients with secondary acute myeloid leukemia (AML) or refractory myelodysplastic syndrome (MDS) enrolled in a Phase I clinical trial of CD8+ depleted allogeneic lymphocyte infusion post-induction chemotherapy.19 The goal was to achieve remission as a bridge to a curative allogeneic HPC transplant by inducing an antitumor response without causing graft versus host disease. Three dose levels were examined (Table 2) and with DL3, two of the 3 patients had complete remissions and proceeded to allogeneic HPC transplant. No patient experienced any dose limiting toxicities.
Looking Ahead
Anticipated Continued Increase in Need for Target Cells
The escalating demand for blood-based cells for the development of gene and cellular therapies is driven by the transformative impact these cells can have on the treatment landscape of various cancers and noncancerous diseases. The versatility of CD34+ cells and other MNCs in gene and cellular therapies is evident in their application across a spectrum of disorders, from genetic deficiencies to hematological malignancies, emphasizing the critical need for efficient and scalable strategies in cell sourcing to meet the burgeoning demands.
Importance of Ongoing Optimization and Establishing High-Quality Standards
Elevating the efficiency and precision of blood-based cell collections demands a comprehensive optimization strategy across multiple parameters. As outlined above, CCS has demonstrated expertise and commitment to continuous improvements that ensure quality-driven, precision cell sourcing in the face of growing demand. These advancements not only enhance the yield and purity of collected cells, but also contribute to the overall efficiency and applicability of gene and cellular therapies, laying the foundation for bringing these effective medical interventions to more patients.
CCS: Ready for the Future
Draw to Thaw™: End-to-End Solution
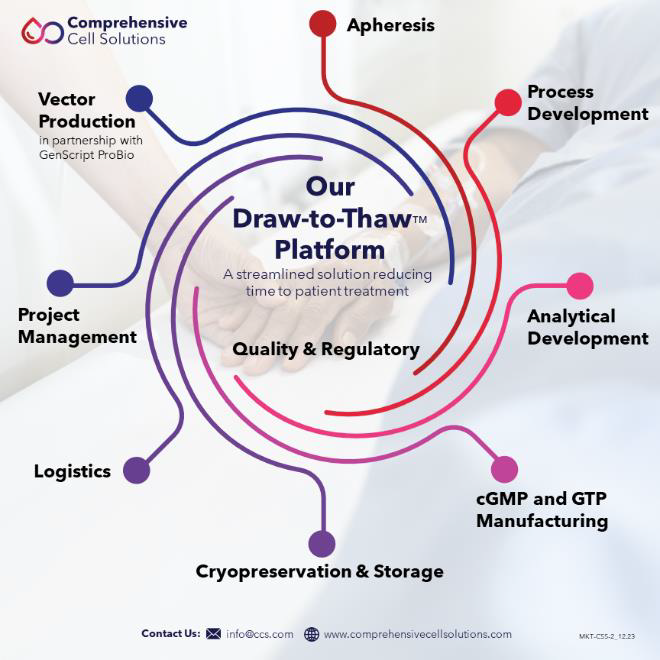
Comprehensive Cell Solutions (CCS), a division of New York Blood Center Enterprises (NYBCe), is an ideal partner for researchers, clinicians, and companies who depend on precise and reliable cell sourcing via apheresis to advance therapies for patients. CCS is focused on pioneering advances in the research, development, and manufacture of cell therapies with end-to-end capabilities (Figure 5). CCS brings over twenty years of experience and expertise in target cell procurement, cryopreservation, and cell processing and manufacturing to collaborations with business and academic partners.
Delivering Excellence, Serving with Purpose
CCS’s legacy as a blood center has positioned it to be a leader in the field of cellular therapy collections. The unmatched collective experience of the CCS team – RNs, PhDs, and MDs with the research and clinical expertise to tailor collections and other processes to a particular donor or patient population of interest – has propelled CCS to the frontline of cell and gene therapy advances that were once thought to be impossible. Over years of collecting all types of blood donors safely, with innovative approaches and quality-driven, validated processes and procedures, CCS is an exemplar in this critical area. Coupled with an unrivaled commitment to patients, CCS is uniquely positioned to meet the growing demands of researchers and manufacturers for reliable, precise, high-quality apheresis products for their downstream investigations or processing.
With its diverse, sizable donor network and collections expertise, CCS is proud to serve as a premier partner to NMDP™ (formerly known as Be the Match), a marrow and stem cell transplantation organization that serves patients around the globe. In addition to dedicated apheresis suites in New York, Rhode Island, and Kentucky, CCS’s team of licensed mobile apheresis nurses and proven logistics extend the reach of these services to the wider community. CCS’ collection and processing facilities have earned accreditation from the Foundation for the Accreditation for Cellular Therapy (FACT) in the areas of hematopoietic cell libraries, immune effector cells, and cord blood banking. FACT accreditation is considered the threshold for excellence in cellular therapy.
As illustrated by our Draw to Thaw™ Platform, apheresed products can move directly into cellular manipulation or manufacturing in our GTP labs and ISO7 clean rooms across facilities in New York and Kentucky. Staff at these facilities have the technical knowledge and expertise to cryopreserve cells or perform complex cell sorting, cell isolation, expansion, and harvest to meet downstream needs after apheresis collection. Bringing together this depth of expertise and breadth of services under one roof streamlines the cell therapy development and manufacturing process, resulting in better outcomes for sponsors and the patients they serve.
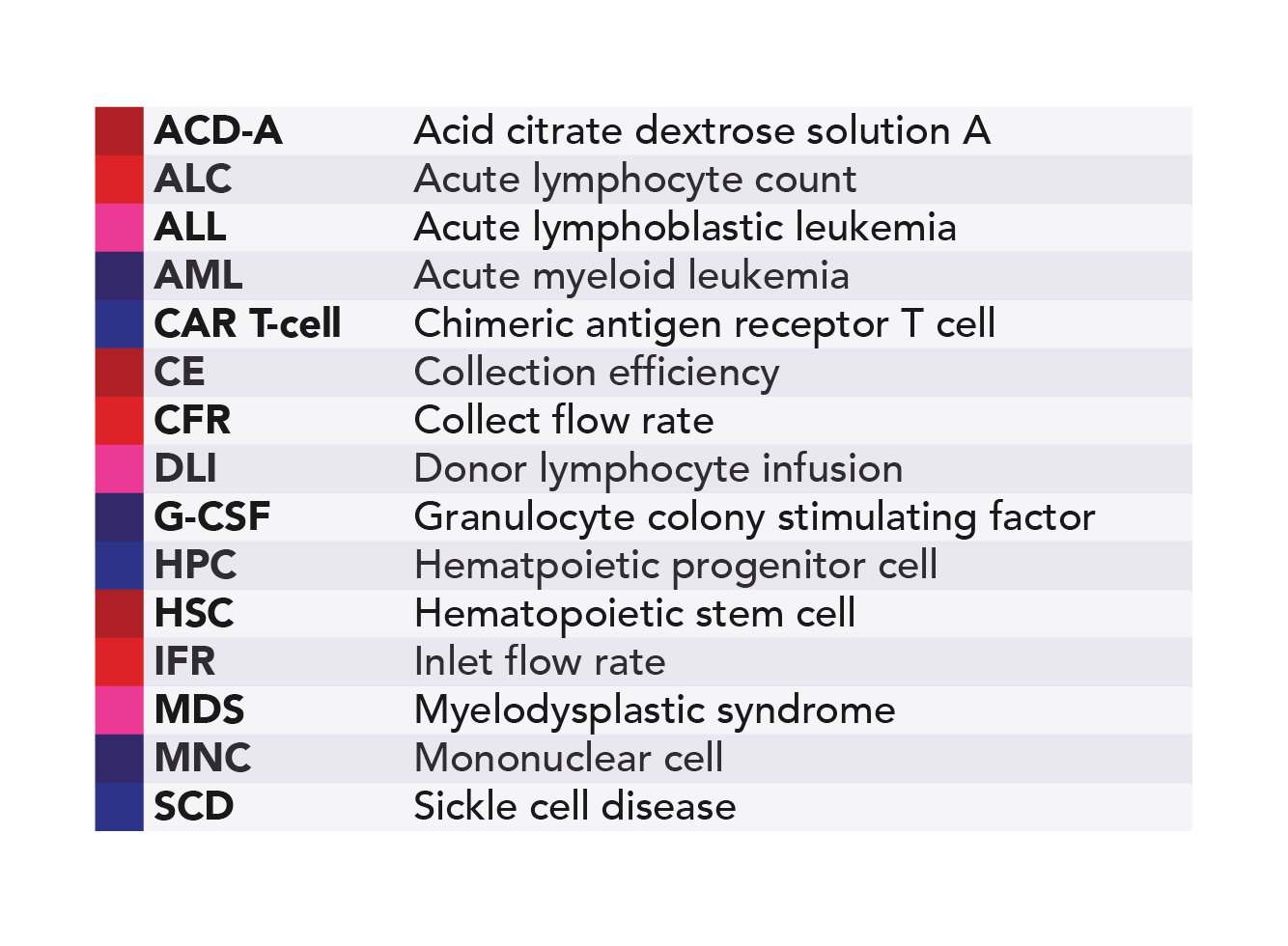
Glossary
References
- Shi PA. Optimizing leukapheresis product yield and purity for blood cell-based gene and immune effector cell therapy. Curr Opin Hematol. 2020;27(6):415-422. doi:10.1097/MOH.0000000000000611
- Bashor CJ, Hilton IB, Bandukwala H, Smith DM, Veiseh O. Engineering the next generation of cell- based therapeutics. Nat Rev Drug Discov. 2022;21(9):655-675. doi:10.1038/s41573-022-00476-6
- Pham HP, Dormesy S, Wolfe K, Budhai A, Sachais BS, Shi PA. Potentially modifiable predictors of cell collection efficiencies and product characteristics of allogeneic hematopoietic progenitor cell collections. Transfusion (Paris). 2021;61(5):1518-1524. doi:10.1111/trf.16370
- Godbey EA, Dormesy S, Gowda L, et al. A dual strategy to optimize hematopoietic progenitor cell collections: validation of a simple prediction algorithm and use of collect flow rates guided by mononuclear cell count. Transfusion (Paris). 2019;59(2):659-670. doi:10.1111/trf.15034
- Yoon EJ, Zhang J, Weinberg RS, et al. Validation of simple prediction algorithms to consistently achieve CD3+ and postselection CD34+ targets with leukapheresis. Transfusion (Paris). 2020;60(1):133-143. doi:10.1111/trf.15576
- Dettke M, Buchta C, Wiesinger H, Maas JH, Strate A, Chen Y. Anticoagulation in large-volume leukapheresis: comparison between citrate- versus heparin-based anticoagulation on safety and CD34 + cell collection efficiency. Cytotherapy. 2012;14(3):350-358. doi:10.3109/14653249.2011.635643
- Shi PA, Luchsinger LL, Greally JM, Delaney CS. Umbilical cord blood: an undervalued and underutilized resource in allogeneic hematopoietic stem cell transplant and novel cell therapy applications. Curr Opin Hematol. 2022;29(6):317-326. doi:10.1097/MOH.0000000000000732
- Karasick MH, Betancourt C, Dormesy S, et al. How do I initiate and maintain a mobile apheresis service in the era of cellular therapy. Transfusion (Paris). 2023;63(1):13-22. doi:10.1111/trf.17143
- Curran KJ, Nikiforow S, Bachier C, et al. A robust quality infrastructure is key to safe and effective delivery of immune effector cells: how FACT-finding can help. Blood Adv. Published online July 19, 2023:bloodadvances.2023010401. doi:10.1182/bloodadvances.2023010401
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- DeSimone RA, Myers GD, Guest EM, Shi PA. Combined heparin/acid citrate dextrose solution A anticoagulation in the Optia continuous mononuclear cell protocol for pediatric lymphocyte apheresis. J Clin Apheresis. 2019;34(4):487-489. doi:10.1002/jca.21675
- Choi E, Branch C, Cui MH, et al. No evidence for cell activation or brain vaso-occlusion with plerixafor mobilization in sickle cell mice. Blood Cells Mol Dis. 2016;57:67-70. doi:10.1016/j.bcmd.2015.12.008
- Boulad F, Shore T, Van Besien K, et al. Safety and efficacy of plerixafor dose escalation for the mobilization of CD34 + hematopoietic progenitor cells in patients with sickle cell disease: interim results. Haematologica. 2018;103(5):770-777. doi:10.3324/haematol.2017.187047
- Pantin J, Purev E, Tian X, et al. Effect of high-dose plerixafor on CD34 + cell mobilization in healthy stem cell donors: results of a randomized crossover trial. Haematologica. 2017;102(3):600-609. doi:10.3324/haematol.2016.147132
- Boulad F, Zhang J, Yazdanbakhsh K, Sadelain M, Shi PA. Evidence for continued dose escalation of plerixafor for hematopoietic progenitor cell collections in sickle cell disease. Blood Cells Mol Dis. 2021;90:102588. doi:10.1016/j.bcmd.2021.102588
- Avecilla ST, Boulad F, Yazdanbakhsh K, Sadelain M, Shi PA. Process and procedural adjustments to improve CD34 + collection efficiency of hematopoietic progenitor cell collections in sickle cell disease. Transfusion (Paris). 2021;61(9):2775-2781. doi:10.1111/trf.16551
- Stone EF, Avecilla ST, Wuest DL, et al. Severe delayed hemolytic transfusion reaction due to anti-Fy3 in a patient with sickle cell disease undergoing red cell exchange prior to hematopoietic progenitor cell collection for gene therapy. Haematologica. 2020;106(1):310-312. doi:10.3324/haematol.2020.253229
- Mendelson A, Liu Y, Bao W, Shi PA. Effect of voxelotor on murine bone marrow and peripheral blood with hematopoietic progenitor cell mobilization for gene therapy of sickle cell disease. Blood Cells Mol Dis. 2024;105:102824. doi:10.1016/j.bcmd.2024.102824
- Elmariah H, Kim J, Reid KM, et al. Phase I trial of CD8-depleted human leukocyte antigen (HLA) mismatched unrelated donor lymphocyte infusion (DLI) to achieve remissions in myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (sAML). Blood. 2022;140(Supplement 1):886-888. doi:10.1182/blood-2022-168175