ABBREVIATIONS:
- ALCs = absolute lymphocyte counts;
- CBCs = complete blood counts;
- CEs = collection efficiencies;
- CFR = collect flow rate;
- DLI = donor lymphocyte infusion;
- HPC = hematopoietic progenitor cell;
- IQR = interquartile range;
- MNC = mononuclear cell;
- PB = peripheral blood;
- RMSE = root mean square error;
- SEs = selection efficiencies;
- TBVs = total blood volumes.
From the 1Clinical Services and 3Lindsley F. Kimball Research Institute, New York Blood Center, New York; 4Division of Pediatric Hematology-Oncology, Northwell Health, New Hyde Park, New York; and the 2Temple University Hospital, Philadelphia, Pennsylvania.
Address reprint requests to: Patricia A. Shi, MD, New York Blood Center 310 East 67th St New York, NY 10128; e-mail: [email protected]
Received for publication May 20, 2019; revision received September 21, 2019, and accepted September 23, 2019.
doi:10.1111/trf.15576
© 2019 AABB
TRANSFUSION 2020;60;133–143
BACKGROUND: Cellular therapies using engineered T cells, haploidentical transplants, and autologous gene therapy are increasing. Specified CD3+ or high CD34+ doses are typically required for subsequent manufacturing, manipulation, or CD34+ selection. Simple, practical, and reliable lymphocyte and hematopoietic progenitor cell (HPC) collection algorithms accounting for subsequent CD34+ selection have not been published.
STUDY DESIGN AND METHODS: In this analysis of 15 haploidentical donors undergoing tandem lymphocyte and HPC collections, we validated one-step, practical prediction algorithms (Appendix S1, available as supporting information in the online version of this paper) that use conservative facility-specific collection efficiencies, CD34+ selection efficiency, and donor-specific peripheral counts to reliably achieve the target CD3+ and CD34+ product doses. These algorithms expand on our previously published work regarding predictive HPC collection algorithms.
RESULTS: Ninety-three percent of lymphocyte and 93% of CD34+ collections achieved the final target CD3+ and CD34+ product dose when our algorithm-calculated process volumes were used. Linear regression analysis of our algorithms for CD3+, preselection CD34+, and postselection CD34+ showed statistically significant models with R2 of 0.80 (root mean square error [RMSE], 31.3), 0.72 (RMSE, 385.7), and 0.56 (RMSE, 326.0), respectively, all with p values less than 0.001.
CONCLUSION: Because achievement of CD3+ or CD34+ dose targets may be critical for safety and efficacy of cell therapies, these simple, practical, and reliable prediction algorithms for lymphocyte and HPC collections should be very useful for collection facilities.
Cellular therapies based on T cells and hematopoietic progenitor cells (HPC), such as engineered T-cell therapy, haploidentical donor hematopoietic stem cell transplants, and autologous gene therapy, are increasing. Reliable means of collecting predetermined amounts of CD3+ and CD34+ cells are crucial for subsequent downstream manufacturing and postcollection manipulation. To our knowledge, no simple, practical leukapheresis collection algorithms have been explicitly published to ensure that 1) specified CD3+ target doses are reached or 2) specified CD34+ target doses are obtained following positive CD34+ selection.
Centers performing collections for cellular therapies may have various approaches for reaching their target doses. Examples include processing a fixed number of liters or total blood volumes (TBVs) in every donor,1,2 or using regression analysis to determine the appropriate volume to process in a given donor.3 The challenges of recipient-specific dose requirements and complicated, nonintuitive analyses, however, may lead to suboptimal collections where target goals are missed.4 As a result, multiple procedures may be necessary,5 which increases recipient risk, financial expense, and donor inconvenience. Ideally, standardized collection algorithms should strike a balance between simplicity, approachability, and accuracy.
In this study, as an extension of our previous work,6 we evaluated the predictive accuracy of practical and approachable algorithms that we devised to calculate the minimum number of donor blood volume liters to process to reach the final target CD3+ and CD34+ doses. We developed these novel algorithms midway through a haploidentical transplant protocol that was new to our collection center for which we were not consistently reaching target doses. The algorithms incorporate center-specific collection efficiencies (CEs) and CD34+ selection efficiencies (SEs) while also accounting for donor-specific cell counts and enumeration. Our retrospective analysis of all patients from protocol inception demonstrates consistent collection of target-cell doses when the predicted number of liters to process is reached.
MATERIALS AND METHODS
Study
The study was conducted under institutional review board approval. Haploidentical donors were collected using a protocol in which a nonmobilized leukapheresis is performed first for a donor lymphocyte infusion (DLI) (target CD3+ dose of 2 × 108 cells/recipient kg).7,8 This is followed by a mobilized leukapheresis to collect an HPC product that subsequently undergoes positive CD34+ selection (target CD34+ dose of 7 × 106 CD34+ cells/recipient kg).
Haploidentical Donors
All 15 haploidentical donors on the protocol were subjected to analysis and were collected between October 2015 and June 2019. DLI collection occurred on Day −7 (7 days before transplant). Donors were mobilized with granulocyte colony stimulating factor on Day −5 for 5 days at 10 μg/kg/day. Peripheral HPC collection occurred on Day −1.
Laboratory Tests
Precollection donor blood (drawn within 1 hour of collection) and samples from the collected products were analyzed for complete blood counts (CBCs) and CD34+ cell counts. CBC and differentials were performed on site using a hematology analyzer (AcT 5 diff AL, Beckman Coulter). CD34+ counts were performed with a single platform method with use of absolute count tubes (TruCOUNT, BD Biosciences) to determine absolute CD45+ and CD34+ counts. Samples were stained with CD45 fluorescein isothiocyanate/CD34+ phycoerythrin monoclonal antibody combination (BD Biosciences) along with 7-amino-actinomycin D and incubated at room temperature in the dark for approximately 15 minutes. Red blood cells (RBCs) were then lysed with use of a lysing solution (PharmLyse, BD Biosciences), and samples were immediately analyzed on a flow cytometer (FACSCalibur, BD Biosciences), with use of the International Society of Hematotherapy and Graft Engineering method for gating. CD3+ enumeration was performed off site with a single platform method with use of a reagent (BD Multitest 6-Color TBNK, BD Biosciences) and TruCOUNT tubes to determine CD3+ absolute counts and percentage of total lymphocytes per the manufacturer’s protocol. In brief, 50 μL of sample was stained with a 6-Color TBNK monoclonal antibody cocktail and incubated at room temperature in the dark for 15 minutes. RBCs were then lysed with a lysing solution (FACS, BD Biosciences) and samples were immediately analyzed on a cytometer (FACSCanto, BD Biosciences).
Collection Procedures
All collections were performed at the main New York Blood Center donor collection site with use of dual-needle peripheral venous access. From 2015 to 2016, seven donors were collected primarily with the mononuclear cell (MNC) collection procedure on an apheresis system (COBE Spectra, version 6.1 or 7, Terumo BCT), whereas from 2017 onward, eight donors were collected with the continuous MNC collection procedure on a different apheresis system (Spectra Optia, version 11.3, Terumo BCT). The COBE Spectra MNC Collect Flow Tool (Caridian BCT, Part No. 306660107) and the Spectra Optia Guidelines for Optimizing the Collect Pump Flow Rate (Terumo BCT, Part No. 777379-061) were used to set the collect flow rate (CFR) for the COBE Spectra6 and Spectra Optia HPC collections, respectively. These tools adjust the CFR based on variation in CBC and inlet flow rate parameters to decrease variability in CE. On-site CBC enumeration allowed for accurate hematocrit programming of the apheresis devices and rapid prediction algorithm calculation for DLI collections. Off-site CD34+ enumeration resulted in approximately 2-hour lag times between the start of collection and prediction algorithm calculation for HPC collections.
CD34+ Selection Procedures
Positive CD34+ selection was performed by the New York Blood Center cell processing facility with a cell-separating device (CliniMACS Plus, Miltenyi Biotec), using up to two vials of CD34+ reagent for each CD34+ selection. CD34+ selection efficiency was defined as the recovered CD34+ dose divided by the CD34+ dose used for selection.
Prediction Algorithms
Prediction algorithms were instituted with the fifth patient for DLI collections and sixth patient for HPC collections after one and two failures to reach target transplant doses with approximately three and five TBVs processed, respectively. Once necessary laboratory tests were resulted, they were input into a protected calculation tool (Excel, Microsoft Corp.) by the covering apheresis physician, and the blood volume to process was relayed by email to the apheresis nurse. If the calculated blood volume to process was very high (>5 TBV) or very low (<3 TBV), the apheresis physician could exercise discretion to adjust the calculated blood volume to process for feasibility or to ensure an adequate collection, respectively.
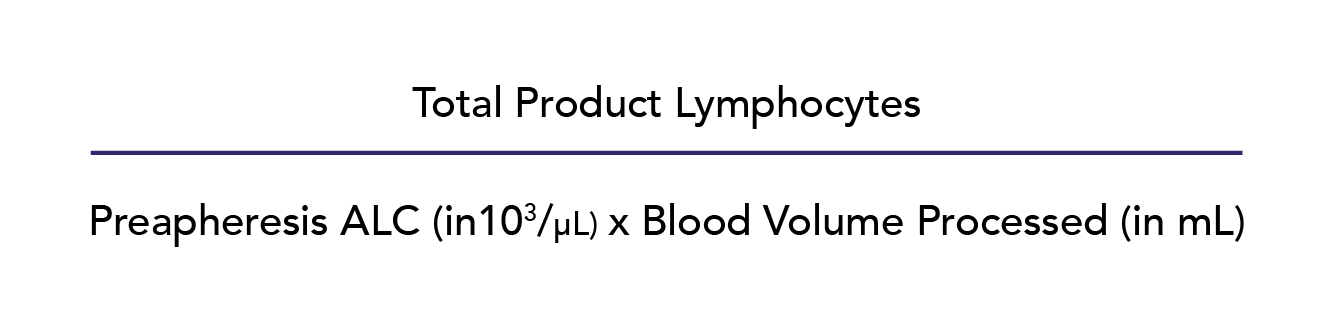
For DLI collections, the donor blood volume to process in liters (Appendix S1, available as supporting information in the online version of this paper, first tab) was calculated as follows:

Donor peripheral blood (PB) absolute lymphocyte counts (ALCs) were used to estimate the corresponding CD3+ concentration, rather than a direct CD3+ enumeration, because ALC measurement is more widely available than CD3+ measurement and less costly to perform. The CD3+ measurement was estimated at 60% of the ALC, which is a conservative estimate of the CD3+ subset of total lymphocytes evident in published literature.9 In addition, our calculations used a conservative first-quartile/25th-percentile lymphocyte CE2 of 0.57 based on historical data. Lymphocyte CE2 was defined as follows:
For HPC collections, the donor blood volume to process in liters (Appendix S1, available as supporting information in the online version of this paper, second tab) was calculated using the donor’s precollection PB CD34+ measurement (pre-CD34+ cells) as follows:
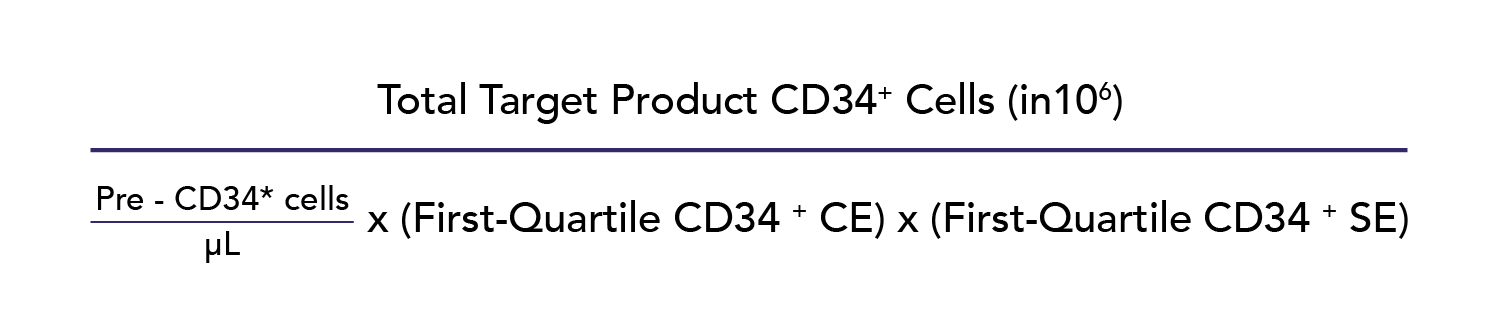
As previously described, our calculations used a conservative historical first-quartile/25th-percentile CD34+ CE2 of 0.40.6 CD34+ CE2 was defined as follows:

Given limited initial experience with positive CD34+ selection with use of the CliniMACS Plus, a conservative SE estimate of 0.30 was used based on the lower end of published ranges.10 Such conservative estimates potentially overestimate the blood volumes to process, which consequently confers the advantage of avoiding undercollection of donors.
Despite use of the above algorithms with donor collection, the actual blood volume processed did not always coincide with the predicted blood volumes to process. (Fig. S1, available as supporting information in the online version of this paper). To account for such discrepancies and estimate what total product counts would be with the target blood volumes processed, the following adjustment was applied:
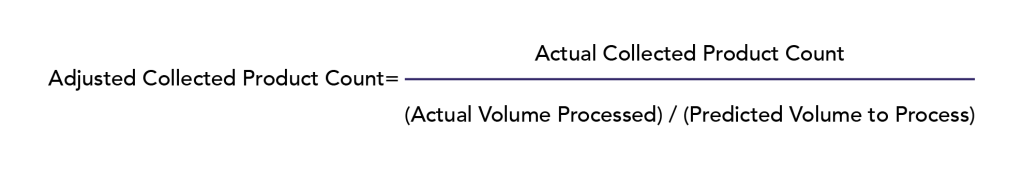
Adjusted collected product counts were then compared to their corresponding initial targets to determine the efficacy of the prediction algorithm for both CD3+ DLI collection (2 × 108 cells/recipient kg) and preselection CD34+ HPC collection (23 × 106 cells/recipient kg, accounting for loss during positive selection). No adjustments were made for CD34+ selection efficiency. Ultimately, the overall efficacy of the CD34+ algorithm accounting for collection and selection was as follows:
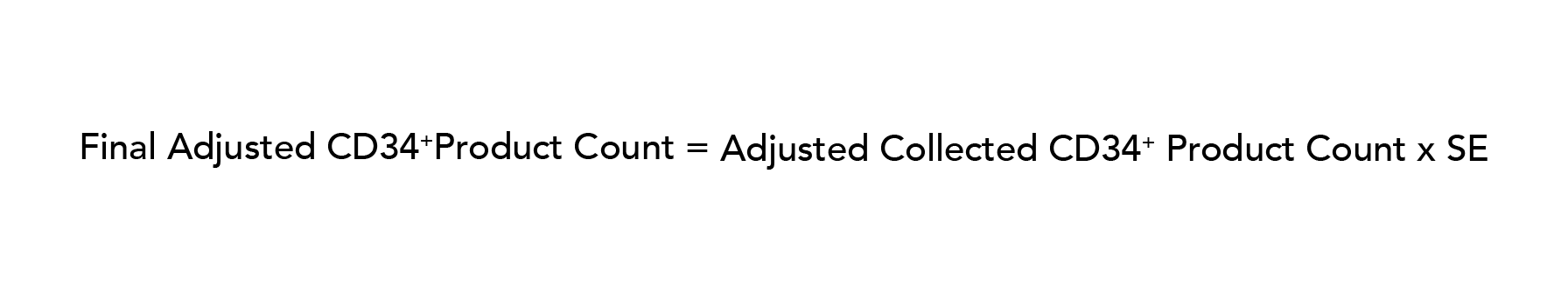
Statistics
Statistical analysis was carried out using computer software (Excel 2010, Microsoft Corp.; and Prism 8.1.0, GraphPad Software). For continuous variables, mean and standard deviation, median and interquartile range (IQR), and Pearson correlation coefficients (r) were generated, and linear regression used to assess the fit of the prediction algorithm models. All statistical tests were assessed with statistical significance set at a p value of 0.05 or less.
RESULTS
Donor Characteristics
Relevant donor characteristics of age, sex, weight, TBV, donor/recipient weight ratio, PB ALC, and PB CD34+ counts are shown in Table 1. All donors were adults and mostly larger than the recipients.
DLI Collection Characteristics
Relevant DLI collection characteristics are shown in Table 2. Over 4 TBV were processed in two of 15 collections. The median ratio of actual/target CD3+ cells collected was 1.63 (IQR, 0.57) with a median actual CD3+ dose collected of 3.26 × 108 (IQR, 1.13 × 108) cells/recipient kg. Actual first-quartile, median, and mean lymphocyte CE2s of 58%, 60%, and 62%, respectively, approximated our first-quartile estimate of 57% and were comparable to a median 55% in prior literature.1 The median and mean CD3+ percentage in collected products of 71% and 71%, respectively, approximated our conservative algorithm prediction of 60%. Two donors (Donors 4 and 5) fell short of the 2 × 108 cells/recipient kg CD3+ collection target at 1.83 and 1.70 × 108 cells/recipient kg, respectively; however, the products were still adequate for transplant (donors were not recollected). The CD3+ percentage in both donors’ products exceeded the 60% predicted by our algorithm; how- ever, the lymphocyte CE2 was lower for both. Donor 4 had a CE of 57% in the context of an actual blood volume processed that was only 68% of the target volume. Donor 5 had a CE2 of 38%, which was well below the prediction even with 97% of the target blood volume processed.
DLI Product Characteristics
Other DLI product (T cells, apheresis) characteristics of interest are shown in Table S1, available as supporting information in the online version of this paper. The mean and median product volumes were higher than other nonstimulated MNC collections,1 at 269 mL and 255 mL, respectively. This can be explained by the larger blood volumes processed in our donors. The median hematocrit of 4.7% was also higher, but this is likely attributable to our use of the Ac·T™ 5 diff AL hematology analyzer as previously reported.6 The median platelet count of 1990 × 103/μL was comparable. In addition, we report a median percentage of granulocyte contamination of 11.0% and a median monocyte percentage of 18.0%.
HPC Collection Characteristics
Relevant HPC collection characteristics are shown in Table 3. Nine of the 15 HPC collections qualified as large-volume procedures, processing over four TBVs each. Five donors (Donors 3-5, 11, and 14) had notably lower blood volumes processed compared to their target volumes due to unfeasibly high targets determined by the collection algorithm. The median ratio of actual/target CD34+ cells collected was 1.01 (IQR, 0.60), while the median actual CD34+ cell dose collected was 23.31 × 106 (IQR, 13.87 × 106) cells/recipient kg. With the target CD34+ collection goal set to 23 × 106 cells/recipient kg to account for a conservatively estimated 30% subsequent SE, seven of the 15 collections fell short of this goal. Notably, in these related donors, actual first-quartile, median, and mean CD34+ CE2 of 37%, 43%, and 44%, respectively, approximated our first-quartile estimate of 40%, but was lower than the median 50% we previously reported with mostly unrelated donors6; this may be an artifact, however, of the larger volume collections. Furthermore, two donors (Donors 9 and 12) had particularly low CE2s attributable to donor-specific peripheral venous access issues, resulting in expectedly inefficient collections.
CD34+ Selection Characteristics
Relevant CD34+ selection characteristics are shown in Table 4. The actual first-quartile SE of 36% approximated our first-quartile estimate of 30%. The median recovered CD34 dose was 9.18 × 106 (IQR 7.72 × 106) cells/recipient kg, and the median ratio of actual recovered/target postselection CD34+ cells was 1.3 (IQR, 1.1). Of note, six donors (Donors 6-10 and 12) had a median 53% (IQR, 44%) of their collected CD34+ product undergo CD34+ selection, due to CliniMACS limits on the total nucleated cell and CD34+ cell numbers that should be placed on the column for a single procedure. The postselection recovery goal of 7 × 106 cells/recipient kg was not met in five of the 15 donors; however, the products were still adequate for transplant (donors were not recollected). Donors 3, 5, 6, and 9 had preselection products well below the 23 × 106 CD34+ cells/recipient kg collection goal, which decreased the likelihood of a sufficient postselection product. Additionally, Donor 14 suffered from both an insufficient preselection CD34+ product and a below-goal SE of 23% due to technical difficulties.
HPC Product Characteristics
Other HPC apheresis product characteristics of interest are shown in Table S1, available as supporting information in the online version of this paper. The median and mean hematocrit of 10.6% and 10.2%, respectively, in preselection products was somewhat higher than we previously reported.6 This is again possibly attributable to the AcT 5 diff AL hematology analyzer.11 Product percentage of granulocyte contamination and platelet counts were similar. Additionally, there was a correlation between the CFR (not shown) and granulocyte contamination (r = 0.70) and was higher with the current large-volume collection than previously noted.6 There was no apparent correlation between the CFR and product hematocrit.
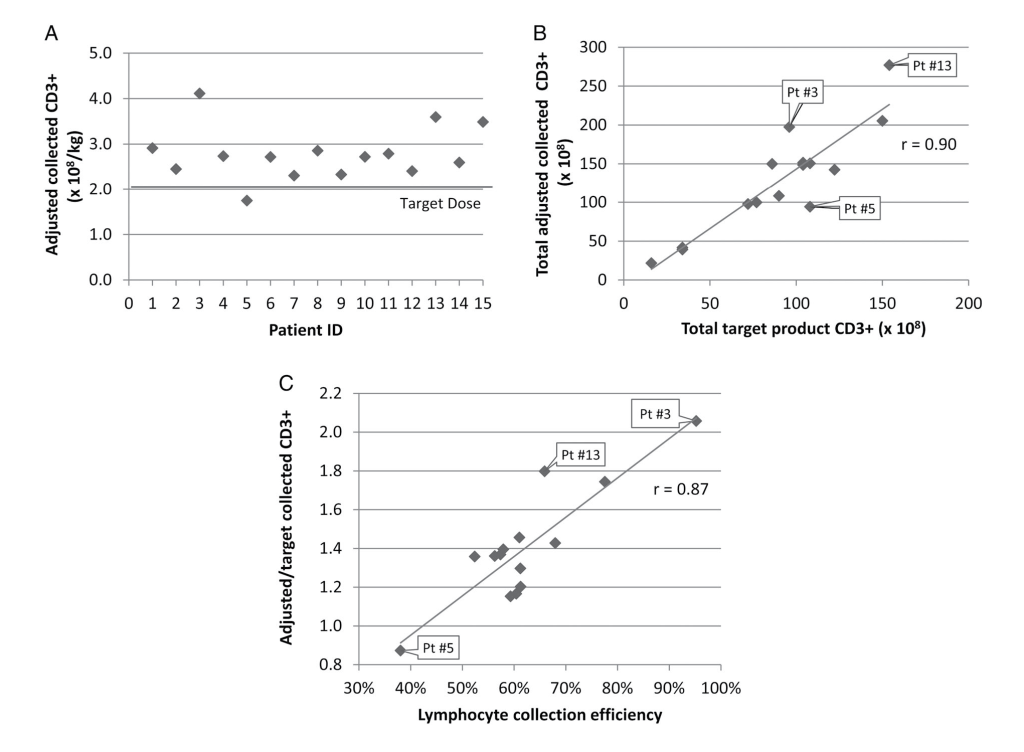
Prediction Algorithm Efficacy
DLI Collection
Three of 15 collections overprocessed greater than 1.5 times the predicted target blood volume due to fear of under- collection, while two collections underprocessed less than 0.75 times the predicted target blood volume (Fig. S1A, available as supporting information in the online version of this paper). Consequently, those products’ CD3+ doses may exceed or fall short of what would have been collected had there been adherence to the predicted blood volume to process. As this may falsely favor or bias against the prediction algorithm, the actual CD3+ dose collected was adjusted (see Methods) to reflect adherence to processing the predicted blood volume (median, 3.02 TBVs; IQR, 1.80). The validity of this adjustment is evidenced by the r = 0.99 correlation between the actual/target volumes processed ratio and the actual/target product CD3+ ratio (Fig. S1B, available as supporting information in the online version of this paper).
This adjustment demonstrated successful collection of the 2 × 108 CD3+ cells/recipient kg target goal in all but one DLI collection (Fig. 1A). The single suboptimal collection had a lymphocyte CE of 38%, notably lower than the 60% CE assumed for the algorithm. The Pearson correlation coefficient between total adjusted and total target product CD3+ dose was strong, at 0.90 (Fig. 1B), with linear regression analysis of the prediction algorithm for the CD3+ target goal resulting in a p value of less than 0.001 (R2, 0.80; root mean square error [RMSE], 31.3). Overall, the median adjusted CD3+ dose collected was 1.36 (IQR, 0.23) times the target goal, with a mean adjusted dose of 1.39 ± 0.29 times the target goal. The r = 0.90 correlation between the total adjusted CD3+ dose collected and the target dose collected was weakened by variation in CEs, with a strong r = 0.87 correlation between the lymphocyte CE and the ratio of adjusted/target CD3+ dose collected (Fig. 1C).
HPC Collection Alone
Five of 15 collections overprocessed greater than 1.2 times the predicted target blood volume due to issues with peripheral CD34+ enumeration turnaround times, which as previously discussed falsely favors the prediction algorithm (Fig. S1C, available as supporting information in the online version of this paper). Conversely, five of 15 procedures underprocessed less than 0.8 times the predicted target blood volume due to unfeasibly high target processing volumes, venous access issues, or early termination due to persistent donor citrate toxicity. Therefore, the actual CD34+ dose collected was adjusted as previously described to reflect adherence to processing the predicted blood volume (median, 4.66; IQR, 5.5 TBV). The r = 0.95 correlation between the actual/target volumes processed ratio and the actual/target product CD34+ ratio validated this adjustment (Fig. S1D, available as supporting information in the online version of this paper).
This adjustment demonstrated successful collection of the 23 × 106 CD34+ cells/recipient kg target goal in 10 of 15 HPC collections (Fig. 2A). The five collections that fell short all had CEs below the 40% CE assumed for the algorithm. The Pearson correlation coefficient between total adjusted and target collected CD34+ count was 0.85 (Fig. 2B), with linear regression analysis of the prediction algorithm for the target CD34+ goal resulting in a p value of less than 0.001 (R2, 0.72; RMSE, 385.7). Overall, the median adjusted CD34+ dose collected was 1.08 (IQR, 0.30) times the target goal with a mean adjusted dose of 1.12 ± 0.29 times the target goal (Fig. 2C). As with the DLI collections, the r = 0.85 correlation between the total adjusted CD34+ dose collected and the target dose collected was weakened by variation in CEs, with a strong r = 1.00 correlation between the CD34+ CE and the ratio of adjusted/target CD34+ dose collected (Fig. 2C).
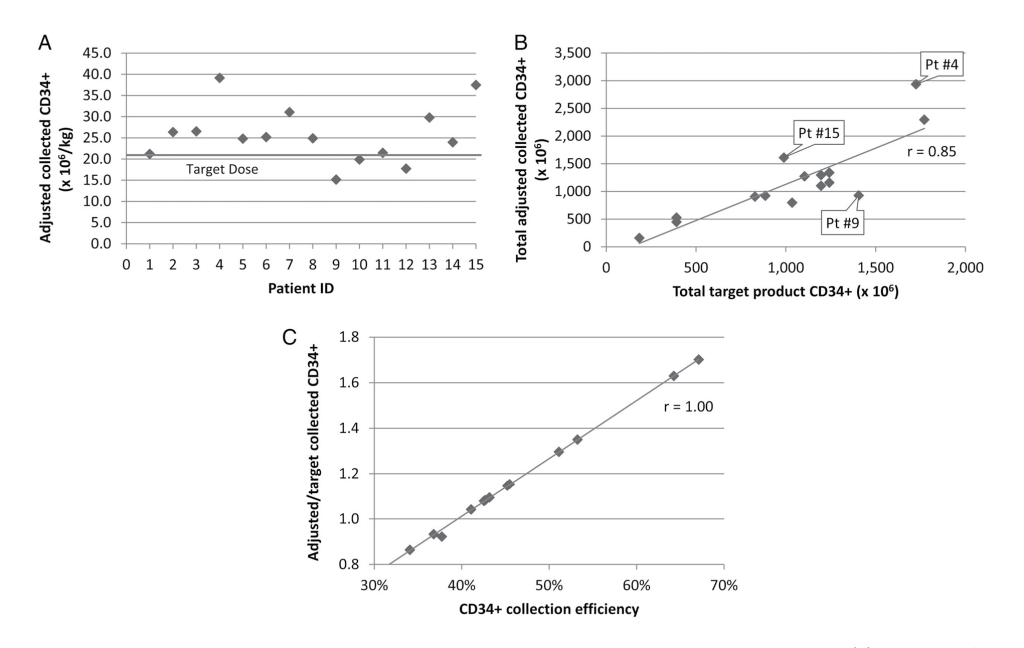
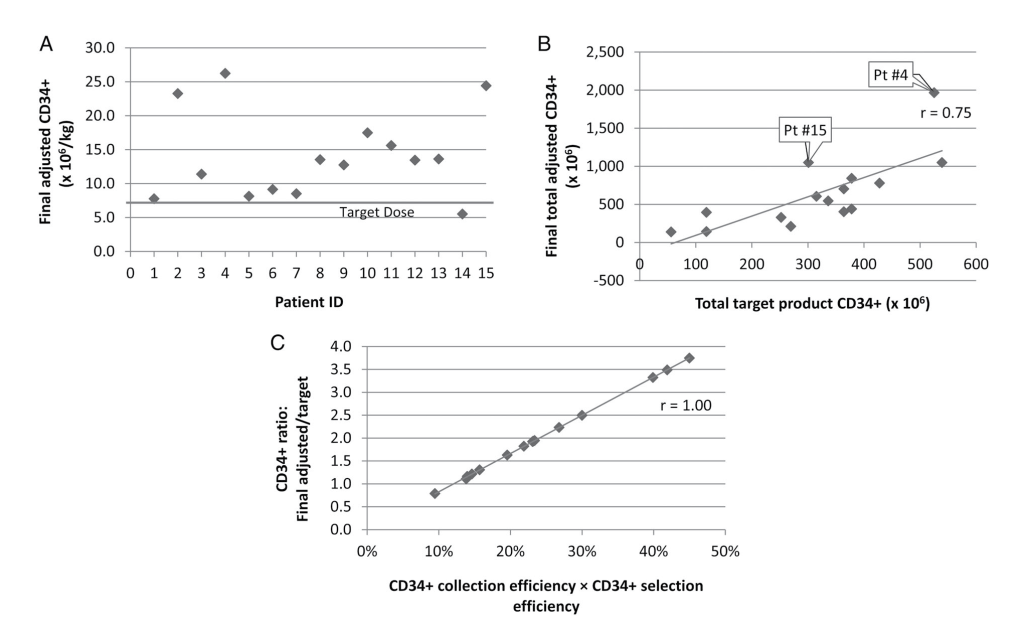
CD34+ Selection Alone
As previously mentioned, the HPC collection algorithm used a conservative SE estimate of 30%10 to calculate blood volumes to process. This minimum SE was achieved in 13 of 15 collections (Table 4).
Combined HPC Collection and CD34+ Selection
The final postselection adjusted target dose of 7 × 106 CD34+ cells/recipient kg was met in all but one collection (Fig. 3A). This single suboptimal collection met the estimated CD34+ CE, but its SE of 23% was the lowest of all selections. The Pearson correlation coefficient between the final postselection adjusted and target collected CD34+ count was 0.75 (Fig. 3B), with linear regression analysis of the prediction algorithm for the target CD34+ goal resulting in a p value of 0.001 (R2, 0.56; RMSE, 326.0). Overall, the median final postselection adjusted CD34+ dose achieved was 1.89 (IQR, 1.11) times the target goal with a mean of 2.00 ± 0.91 times the target goal (Fig. 3C). The r = 0.75 correlation between the final post- selection adjusted CD34+ dose and the target dose was weakened by the combined effects of variation in CD34+ CEs and SEs. There was a strong r = 1.00 correlation between the ratio of the final postselection adjusted/target CD34+ dose and the product of CD34+ CE and SE (Fig. 3C).
DISCUSSION
Leukapheresis collection for the expanding indications for CD3+ and CD34+ cell-based therapies continues to be a challenging endeavor. Our CD3+ and CD34+ cell collection algorithms were first presented in 201612 and represent, to our knowledge, the first validations of intuitive, easily implemented algorithms that factor in CD34+ selection and CD3+ lymphocyte percentage estimation. The included Excel format fillable calculation tools (File S1, available as supporting information in the online version of this paper) have been validated to correspond with manual calculations. To prevent accidental changes, the spreadsheet cells that incorporate mathematical formulas and the spreadsheet itself have been locked by the institution’s medical director. At our institution, each instance of volume calculation using this tool is performed directly by the apheresis physician, or the tool is populated by designated trained staff members, with sign-off by the apheresis physician. The Excel files are currently in the process of being added to our formal controlled document system that has version control.
When actual product counts were adjusted for target volume to process, 93% of both DLI and HPC collections reached the final target CD3+ and CD34+ doses, respectively. Importantly, our CD34+ collection algorithm differs from the one we previously published6 and, to our knowledge, from any others by uniquely accounting for efficiency of CD34+ positive selection. Also of importance, our CD3+ collection algorithm differs from others published by using peripheral blood absolute lymphocyte count to estimate CD3+ count rather than a more generic MNC count13 or a measured CD3+ cell count. An online tool similar to our CD3+ collection algorithm is now publicly available14 but provides no validated method to estimate CD3+ lymphocyte percentage or collection efficiency.
Although others have also published leukapheresis collection prediction algorithms, our algorithms calculate the donor blood volume to process based on the target dose, rather than using a relatively fixed blood volume to process regardless of target dose.1,2 Other algorithms calculating donor volume to process used linear regression,3 which is more mathematically complex, possibly sufficiently unintuitive to deter potential users, and, most importantly, can result in undercollection. Although our algorithms may predict a target volume to process that is unfeasibly high, it is useful for planning and considerate to the donor to know as early as possible whether additional collection days may be necessary. Processing as much target volume as feasible facilitates achieving the target dose with the least possible number of leukapheresis procedures, thus optimizing donor safety and convenience.
Our study, like many others,15,16 demonstrated significant variation in collection efficiency and selection efficiency. Factors that may affect CE include the inlet flow rate and preprocedure target cell count (factored in by CFR adjustment), the target cell type, volumes processed, interface stability, venous access issues, apheresis device used, and fac- tors intrinsic to the specific donor, including whether they are autologous or allogeneic.17,18 Perhaps of interest, the only DLI collection that did not meet the target due to a lymphocyte CE of only 38% was from the oldest donor (age 60) and had the highest percentage of granulocytes. Interrogation of the two HPC collections with the lowest CEs revealed multiple inlet and return pressure alarms; the CE estimate could be adjusted downward in such cases. Regarding SE variability, CD34+ selection using the CliniMACS Plus is processing step dependent19; lower variability with CD34+ selection is possible using the highly automated CliniMACS Prodigy (Miltenyi Biotec), but at the cost of a lower average selection efficiency.16
Given variations between centers in apheresis and processing practices, we recommend that institutions use their own historical data-based, device-specific, procedure-specific, and donor-specific, conservative (25th percentile) CE and SE values in our algorithms, to maximize the probability of collection and selection procedures successfully achieving goals. Although this approach has a higher potential for over- collection of donors, this is preferable to undercollection with the need for additional collection attempts to reach target goals. Estimating a lower CE may also be beneficial in cases where processing large volumes is necessary, such as in donors who are smaller than their recipients, because, although less likely with allogeneic donations,20 CE may decrease during collection as the target cell population is depleted.21
Fourteen of 15 DLI collections (93%) reached target with use of the predicted volumes to process while over-collecting a median 36% (median adjusted CD3+ dose collected, 1.36 [IQR, 0.23] times the target goal). While only 10 of 15 HPC collections (67%) reached their preselection target goal using predicted volumes to process, concurrent use of a conservative 30% SE estimate for CD34+ selection ultimately resulted in 14 of 15 collections (93%) successfully reaching their postselection target goal. Prior to CD34+ selection, the median adjusted CD34+ dose collected was only 1.08 (IQR, 0.30) times the target goal, whereas after selection, the median adjusted CD34+ dose achieved was 1.89 (IQR, 1.11) times the target goal.
There are some limitations to our study. First, all donors from our study were adult related donors, and some studies suggest that CD34+ CEs may differ in allogeneic compared to autologous donors.3,17 However, provided that centers use CEs specific to their autologous versus allogeneic donor populations, it is reasonable to presume that our predictive algorithms could be applied in other contexts such as autologous or unrelated donor cellular collections for T-cell–based therapy or autologous gene therapy. Indeed, our correlation coefficients between the adjusted actual and target final product doses in the current study were 0.90 and 0.75 for CD3+ and CD34+, respectively, similar to that for CD34+ of 0.78 in our previous allogeneic unrelated donor study.6 Second, donor PB ALC and CD34+ counts were available to calculate CE2, which uses only donor precollection counts, but not CE1, which utilizes an average of donor precollection and postcollection counts. CE2 may overestimate or underestimate22,23 actual CEs. Finally, early collections (12 of 30 procedures) were performed on the COBE Spectra rather than the currently supported Spectra Optia; however, the advantage of the Optia relates to a possibly higher CE and lower hematocrit, platelet, and granulocyte contamination.15,24 These fac- tors should not affect the efficacy of our algorithms when adjusted for the center-specific (and thus device-specific) CE.
In conclusion, our simple, easy-to-implement predictive algorithms incorporating center-specific, conservative procedural efficiencies and donor-specific peripheral blood parameters allow collection centers to reliably attain target cell doses and thus streamline collections for advanced cellular therapies, improving overall experiences for donors and recipients.
ACKNOWLEDGMENTS
The authors thank the donors who were the subjects of this study and the excellent apheresis nurses for their contribution to improved patient care.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- Putensen D, Smith R, Pilcher L, et al. Comparison of the CMNC and MNC apheresis protocol for the collection of T-cells showed comparable outcome: an observational study in a single centre. J Clin Apher 2018;33:349-56.
- Bartnik K, Maciejewska M, Farhan R, et al. Continuous mononuclear cell collection (cMNC) protocol impact on hematopoietic stem cell collections in donors with negative collection predictors. Transfus Apher Sci 2018;57:401-5.
- Leberfinger DL, Badman KL, Roig JM, et al. Improved planning of leukapheresis endpoint with customized prediction algorithm: minimizing collection days, volume of blood processed, procedure time, and citrate toxicity. Transfusion 2017;57:685-93.
- Allen ES, Stroncek DF, Ren J, et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion 2017;57:1133-41.
- Huenecke S, Bremm M, Cappel C, et al. Optimization of individualized graft composition: CD3/CD19 depletion combined with CD34 selection for haploidentical transplantation. Transfusion 2016;56:2336-45.
- Godbey EA, Dormesy S, Gowda L, et al. A dual strategy to optimize hematopoietic progenitor cell collections: validation of a simple prediction algorithm and use of collect flow rates guided by mononuclear cell count. Transfusion 2019;59:659-70.
- Gaballa S, Palmisiano N, Alpdogan O, et al. A two-step haploidentical versus a two-step matched related allogeneic myeloablative peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2016;22:141-8.
- Grosso D, Gaballa S, Alpdogan O, et al. A two-step approach to myeloablative haploidentical transplantation: low nonrelapse mortality and high survival confirmed in patients with earlier stage disease. Biol Blood Marrow Transplant 2015;21:646-52.
- Valiathan R, Deeb K, Diamante M, et al. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology 2014;219:487-96.
- Watts MJ, Somervaille TC, Ings SJ, et al. Variable product purity and functional capacity after CD34 selection: a direct comparison of the CliniMACS (v2.1) and Isolex 300i (v2.5) clinical scale devices. Br J Haematol 2002;118:117-23.
- Avecilla ST, Marionneaux SM, Leiva TD, et al. Comparison of manual hematocrit determinations versus automated methods for hematopoietic progenitor cell apheresis products. Transfusion 2016;56:528-32.
- Yoon EJ, Weinberg RS, Sachais BS, Brochstein JA, Shi PA. Collection and Processing considerations in haploidentical stem cell transplantation utilizing a 2-step methodology. American Society of Hematology Annual Meeting Orlando, FL: Blood, 2016:2178.
- Ceppi F, Rivers J, Annesley C, et al. Lymphocyte apheresis for chimeric antigen receptor T-cell manufacturing in children and young adults with leukemia and neuroblastoma. Transfusion 2018;58:1414-20.
- Hauser RG, Cheng C, Tormey C. Donor lymphocyte infusion (DLI) volume, MDCalc.com. Available from: https://www. mdcalc.com/donor-lymphocyte-infusion-dli-volume. Accessed September 20, 2019.
- Spoerl S, Wascher D, Nagel S, et al. Evaluation of the new continuous mononuclear cell collection protocol versus an older version on two different apheresis machines. Transfusion 2018;58:1772-80.
- Stroncek DF, Tran M, Frodigh SE, et al. Preliminary evaluation of a highly automated instrument for the selection of CD34+ cells from mobilized peripheral blood stem cell concentrates. Transfusion 2016;56:511-7.
- Cousins AF, Sinclair JE, Alcorn MJ, et al. HPC-A dose prediction on the optia(R) cell separator based on a benchmark CE2 collection efficiency: promoting clinical efficiency, minimizing toxicity, and allowing quality control. J Clin Apher 2015;30: 321-8.
- Humpe A, Riggert J, Meineke I, et al. A cell-kinetic model of CD34+ cell mobilization and harvest: development of a predictive algorithm for CD34+ cell yield in PBPC collections. Trans- fusion 2000;40:1363-70.
- Panch SR, Reddy OL, Li K, et al. Robust selections of various hematopoietic cell fractions on the CliniMACS plus instrument. Clin Hematol Int 2019;1:161-7.
- Verlinden A, Van de Velde A, Verpooten GA, et al. Determining factors predictive of CD34+ cell collection efficiency in an effort to avoid extended and repeated apheresis sessions. J Clin Apher 2013;28:404-10.
- Ford CD, Greenwood J, Strupp A, et al. Change in CD34+ cell concentration during peripheral blood progenitor cell collection: effects on collection efficiency and efficacy. Transfusion 2002;42:904-11.
- Fontana S, Groebli R, Leibundgut K, et al. Progenitor cell recruitment during individualized high-flow, very-large-volume apheresis for autologous transplantation improves collection efficiency. Transfusion 2006;46:1408-16.
- Humpe A, Riggert J, Koch S, et al. Prospective, randomized, sequential, crossover trial of large-volume vs. normal-volume leukapheresis procedures: effects on subpopulations of CD34 (+) cells. J Clin Apher 2001;16:109-13.
- Cancelas JA, Scott EP, Bill JR. Continuous CD34+ cell collection by a new device is safe and more efficient than by a standard collection procedure: results of a two-center, crossover, randomized trial. Transfusion 2016;56:2824-32.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article.
Appendix S1: Supplementary material
Table S1: Other relevant product characteristics
Fig. S1: Rationale and validity of CD3+ and CD34+ product dose adjustment to evaluate prediction algorithms. The prediction algorithm–based target volume to process did not always match the actual volume processed for (A) CD3+ and (C) CD34+ collections. Therefore, the actual collected product count was adjusted to reflect what would have been collected had the target volume been processed. The validity of the adjustment is evident by the high correlation coefficients between the ratios of actual to target volume processed and actual/target cell dose for (B) CD3+ and (D) CD34+.