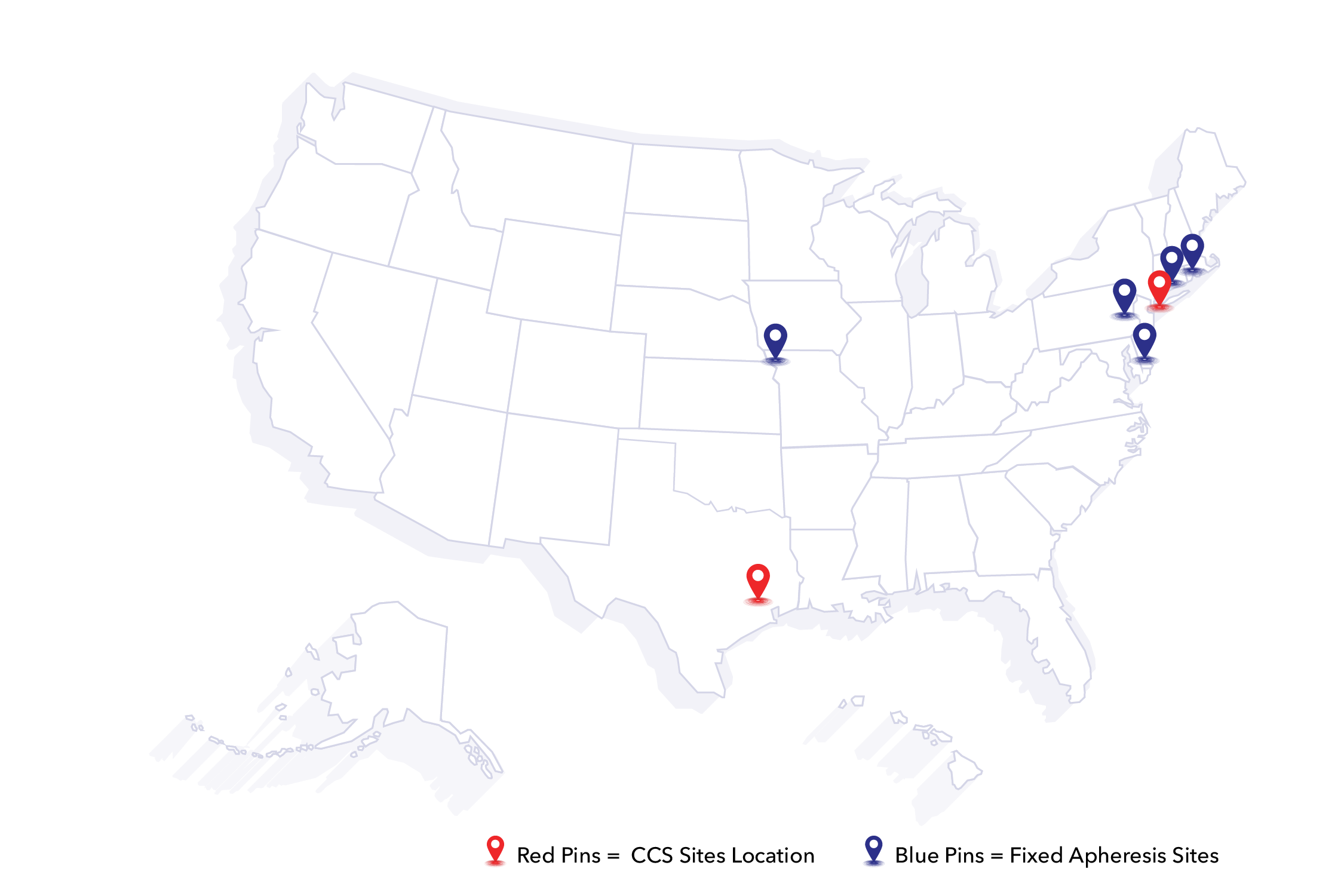
Locations
Nationwide Facilities
Delivering Scalable
Solutions
Locations
With strategically placed manufacturing and cell collection sites, CCS delivers cellular starting material, process and analytical development, and GMP/GTP services with unmatched efficiency and accessibility.
- Rye, NY: Flagship location for cell sourcing, stem cell technology, and cord blood-derived stem cells.
- Houston, TX: Process development and analytical testing hub, supporting early-phase assets and pre-clinical assets.
With our Draw-to-Thaw™ solutions and seamless technology transfer process, CCS ensures efficient logistics and scalable cell therapy solutions to meet your needs.
Licenses, Permits & Certificates
As a business unit of New York Blood Center Enterprises, we are routinely inspected and licensed based on the operations we perform and the states in which we operate. Our FACT-accredited facilities and CLIA-certified laboratories and personnel uphold the highest standards that govern our industry. For copies of our regulatory licenses for specific locations and services, please visit our NYBC reference page.